Application: MicroPulseTM Diode Laser
Trabeculoplasty (MDLT)
Products: IRIS Medical® Infrared (810 nm) Laser Photocoagulators Slit
Lamp Adapter
Clinical Series: MicroPulse Diode Laser Trabeculoplasty versus Argon Laser
Trabeculoplasty in the Treatment of Open Angle Glaucoma
Ingvoldstad DD, Krishna R, Willoughby L. Ophthalmology, University of
Missouri, Kansas City, MO. Presented at ARVO 2005, Poster 123 |
- Review & Rational: Giorgio Dorin, IRIDEX Corporation, Mountain
View, CA.
Photothermal Laser Trabeculoplasty Laser trabeculoplasty
(LT) was first described in 1974 by Worthen and Whickham.1
Following the publication of Wise and Witter’s pilot study in 1979,2
photothermal LT with either argon lasers (ALT) or diode lasers
(DLT) became a commonly performed procedure for the treatment of primary
open-angle glaucoma (POAG), exfoliation syndrome glaucoma, pigmentary
glaucoma and normal-tension glaucoma.
The effectiveness and the role of LT in various phases of the management
of open angle glaucoma (OAG) have been defined by landmark multicenter
National Eye Institute (NEI)-sponsored trials: the Glaucoma Laser Trial
(GLT);3 the Advanced Glaucoma Intervention Study (AGIS);4
the Collaborative Initial Glaucoma Treatment Study (CIGTS);5-6
and the Early Manifest Glaucoma Trial (EMGT).7-8
In 1996, on the basis of the evidence from the Glaucoma Laser Trial
Follow-up Study (GLTFS),3 the NEI reported that ALT is at
least as efficacious as medications as first-line therapy in newly diagnosed
POAG; however, LT was not generally adopted as the primary treatment
of choice and was mainly used as an adjunct therapy reserved for cases
of uncontrolled IOP with maximum tolerated medications. Only in few
selected cases, LT was used as an initial treatment (older patients,
patients unable to use beta-adrenergic antagonists or poorly compliant
with medications) or as an alternative to miotics and/or carbonic anhydrase
inhibitors to reduce patients’ discomfort and side effects.
The introduction of new pharmaceutical agents further shifted the use
of LT, which, although proved superior than medications-first in a randomized
trial in the UK9 and considered by some a cost-effective initial treatment
of choice for chronic OAG,10-11 gradually became a relatively
underused therapy, probably due to: Review & Rational: Giorgio Dorin,
IRIDEX Corporation, Mountain View, CA.
- new advances in medical therapy
- fading long-term efficacy (decreasing effects in 50% of patients after
5 years)
- lower effectiveness in younger patients (LT works best in patients
over 60 years of age)
- limited ability to retreat
- undesirable side effects and collateral damage, mostly caused by the
laser protocols used for ALT and DLT, such as
- post-op inflammatory response
- acute and in few cases chronic IOP elevation
- peripheral anterior synechiae (PAS)
- scarring of the TM
In essence, LT, as administered with current ALT and DLT protocols,
is a proven and cost-effective glaucoma therapy, whose usage however
is limited by the concerns over iatrogenic acute and chronic photothermal
damage.
Selective Laser Trabeculoplasty To minimize the iatrogenic
damage of LT, Latina introduced the concept of selective laser trabeculoplasty
(SLT) in 1995. SLT uses a 532 nm Q-switched frequency-doubled Nd:YAG
laser emitting short pulses to limit the destruction to the trabecular
meshwork (TM) pigment-laden cells, in accordance with the principles
of selective laser photothermolysis. Indeed SLT has demonstrated isolated
destruction of pigmented TM cells without collateral damage to surrounding
non-pigmented cells. SLT protocol uses pulse energy of 0.8-1.0 mJ delivered
in a single 3ns pulse at a very high peak power (˜ 0.3 megawatts).
Despite the relatively large 400 µm spot, this peak power translates
in a very high irradiance (265 megawatts/ cm2) that produces micro-explosions
(the champagne-like bubbles endpoint), which unfortunately cause acute
anterior chamber (AC) reactions and post-op IOP spikes requiring postop
steroid therapy. IOP lowering was found equally effective as with ALT
in a number of clinical studies, and in March 2001, SLT received
the PMA by the FDA.
Interestingly, the average percentage of IOP reduction reported by the
great majority of studies with SLT, ALT, and DLT has been comparable
and consistently within the 20-25% range, regardless of the LT technique/protocol
used.
The efficacy and the success rate of LT appear to be more dependent
on patients’ factors (race, age, pigmentation, pre-op IOP, stage of
glaucoma and fellow eye response to laser) than on the LT technique/protocol.
Thus, the least possible damaging LT technique/protocol could have the
best chance to gaining the acceptance by the ophthalmic community and
to enhance LT’s role and time of intervention in the sequence of glaucoma
management. Glaucoma is a 24 hours/day degenerative disease in which
each single mm Hg counts: a successful, long-lasting or repeatable LT
could constitute a cost-effective therapy with the added benefit of
blunting diurnal IOP variations.12
Micropulse Diode Laser Trabeculoplasty (MDLT)
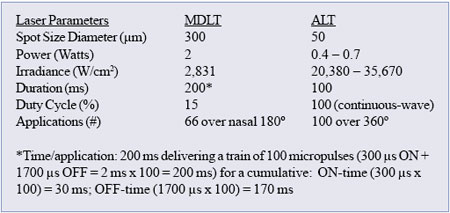
MDLT is a large-spot, low-irradiance minimum intensity photocoagulationTM
(MIP) protocol for the reduction of IOP with a non-destructive laser/tissue
interaction to minimize collateral cell damage. MDLT utilizes trains
of short 810 nm laser pulses (100-300 µsec) to control and spatially
confine the laser-induced thermal elevation in order to produce sublethal,
photothermal cellular effects only around the TM pigmented cells.
MDLT is typically performed delivering 66 confluent 300 µm diameter
invisible laser applications covering the whole height of the TM over
180°. Due to the combination of a) low absorption of the 810 nm laser
wavelength by the TM, b) the relatively large 300 µm spot at low irradiance
(2 W over 300 µm spot = 2,830 W/ cm2), MDLT interacts with most pigmented
cells in the superficial and deep layers of the TM without producing
visible effects, tissue blanching, or bubble formation. The treatment
is invisible to the surgeon and uneventful for the patient with no thermal
pain and no laser flashes (810 nm is invisible). The eye remains quiet
with negligible or no flare, no visible post-op inflammation, no IOP
spikes and no need for post-op steroids therapy.
In a randomized pilot study conducted at the University of Missouri
Kansas City,13 MDLT and ALT showed an equal IOP lowering
effect at 3 months. IOP reduction from baseline was statistically significant
for both study’s arms with no difference between arms. At 1-hour from
treatment, cell and flare reaction at “trace-1+” level was found in
10/11 (91%) of the ALT eyes and in 2/10 (20%) of the MDLT eyes.
These early results seem to support the hypotheses that all forms of
LT may share a same cellular mechanism of action. Unlike other modalities,
MDLT interacts but does not destroy pigmented endothelial cells in the
TM, and reaches the unknown thresholds of cytokines activation and MMP-3
upregulation without visible photothermal damage and inflammatory reactions.
The rationale for MDLT can be summarized as follows:
- DLT works. It has shown an IOP lowering effect equivalent to ALT,14
with less inflammatory reaction, less disruption of the blood
-aqueous barrier, no postoperative pain, and no PAS.15
- MDLT is a lower dose DLT that uses lowirradiance micropulsing to allow
better control and confinement of the photothermal effects16
- 17 to minimize the damage to the TM.
- Less TM damage with no scarring would theoretically minimize the risk
for late IOP rise and offer the potential for retreatments and/or staged
periodical enhancements.
- MDLT is painless and has no reported complications (eyes are quiet
with negligible inflammation, no IOP spikes, no PAS).
- MDLT is easy to perform: the large 300 µm spot is less critical to
aim and focus than the smaller spots used with ALT and DLT.
- MDLT, unlike SLT, does not require a dedicated expensive new laser.
MDLT is performed with the IRIS Medical SLx OcuLight 810 nm photocoagulator,
a multipurpose ophthalmic laser that is readily available to the glaucoma
specialist performing DLT or transscleral cyclophotocoagulation with
the G-ProbeTM and that can be found in many ophthalmic departments where
is routinely used for transpupillary, transscleral and intraocular treatments
of several ocular disorders in the office or in the operating room.
|
References
1. Worthen DM, Wickam MG. Argon laser trabeculotomy. Trans Am Acad Ophthalmol
Otolaryngol 1974;78:OP371-OP375.
2. Wise JB, Witter SL. Argon laser therapy for open angle glaucoma: a pilot
study. Arch Ophthalmol 1979;97:319- 322.
3. Glaucoma Laser Trial Research Group. The Glaucoma Laser Trial (GLT) and
Glaucoma Laser Trial Follow-up Study:7. Results. Am J Ophthalmol 1995;120:718-731.
4. The Advanced Glaucoma Intervention Sudy 13. Comparision od treatment
outcomes within race 10 years results. Ophthalmology 2004;111:651-664.
5. Musch DC et al for the CIGTS Study Group. The collaborative initial glaucoma
treatment study. Study design, methods, and baseline characteristics of
enrolled patients. Ophthalmology 1999;106:653-662.
6. Lichter PR et al for the CIGTS Study Group. Interim clinical outcomes
in the collaborative initial glaucoma treatment study comparing initial
treatment randomized to medications or surgery. Ophthalmology 2001;108:1943-1953.
7. Heijl a et al for the Early Manifest Glaucoma Trial Group. Reduction
of intraocular pressure and glaucoma progression. Results from the Early
Manifest Glaucoma Trial. Arch Ophthalmol 2002;120:1268-1279.
8. Leske MC et al for the Early Manifest Glaucoma Trial Group. Factors for
glaucoma progression and effect of treatment. The Early Manifest Glaucoma
Trial. . Arch Ophthalmol 2003;121:48-56.
9. Migdal C, Gregory W, Hitchings R. Long-term functional outcome after
early surgery compared with laser and medicine in open-angle glaucoma. Ophthalmology
1994; 101:1651-1656
10. Jampel HD et al. Initial treatment for open-angle glaucoma-Medical,
laser, or surgical? Arch Ophthalmol 1998;116:239-242.
11. Migdal C, Hitchings R. Primary therapy for chronic simple glaucoma.
The role of argon laser trabeculoplasty. Transactions of the Ophthalmological
Societies of the United Kingdom. 62-66.
12. Greenidge KC, Spaeth GL, Fiol-Silva Z. Effect of argon laser trabeculoplasty
in the glaucomatous diurnal curve. Ophthalmology 1983;90:800-804.
13. Ingvoldstad DD, Krishna R, Willoughby L. Micropulse Diode Laser Trabeculoplasty
versus Argon Laser Trabeculoplasty in the treatment of Open Angle Glaucoma.
Invest Ophthal Vis Sci 2005;46:ARVO E-Abstract 123.
14. Brancato R, Carassa R,Trabucchi G. Diode laser compared with argon laser
for trabeculoplasty. Am J Ophthalmol 1991;112:50-55.
15. Moriarty a, McHugh D, Spalton D, Ffytche T, Shah S, Marshall J. Comparison
of the anterior chamber inflammatory response to diode and argon laser trabeculoplasty
using a laser flare meter. Ophthalmology 1993; 100:1263-1267.
16. Mainster MA. Decreasing retinal photocoagulation damage: principles
and techniques. Semin Ophthalmol 1999;14(4):200-209.
17. Dorin G. Subthreshold and micropulse diode laser photocoagulation. Semin
Ophthalmol 2003;18(3):147-143. |
 Список
статей
Список
статей