 INDOCYANINE
GREEN-ENHANCED TRANSPUPILLARY THERMOTHERAPY OF CHOROIDAL MELANOMA INDOCYANINE
GREEN-ENHANCED TRANSPUPILLARY THERMOTHERAPY OF CHOROIDAL MELANOMA
Choroidal melanoma is the most common intraocular malignant tumor of the
eye in the adult population. Transpupillary thermotherapy (TTT) has been
used successfully in the treatment of choroidal melanomas.1-5 During TTT,
near-infrared radiation energy (810 nm) from a diode laser is delivered
to the tumor through a dilated pupil. Experimental models show that the
temperature of the tumor increases to 45 - 60°C (intermediate-level hyperthermia)
with an exposure of 1 minute or more.6 These levels of hyperthermia produce
a direct cytotoxic effect (tumoricidal phenomena) on tumor cells.
Indocyanine green (ICG) is a water-soluble anionic dye approved by the
U.S. Food and Drug Administration for indocyanine angiography in ophthalmology
decades ago.7 ICG is safe, inexpensive, and associated with only isolated
case reports of adverse reactions.8 ICG exhibits an absorption spectrum
in the infrared band. In experimental models9-11 and clinical studies
of choroidal melanoma, this molecule in combination with an infrared diode
laser used in a thermal mode acts synergistically to almost double the
tissue destruction effect from TTT when used alone.12 ICG, when injected
prior to a TTT treatment, acts as a chromophore enhancing the photothermal
effect and as a fluorophor producing photochemical effects resulting in
a photodynamic therapy reaction (i-TTT).
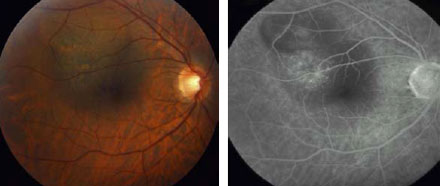 Fig 1c. Red free fundus
Fig 1c. Red free fundus
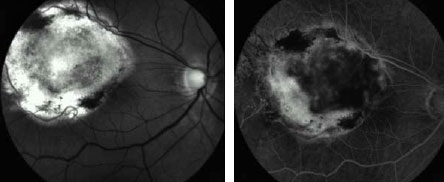 Fig 1d. Middle FA frame 3 months after the second session disclosed
residual tissue without evidence of activity. BCVA: 20/60.
Fig 1d. Middle FA frame 3 months after the second session disclosed
residual tissue without evidence of activity. BCVA: 20/60.
We evaluated 25 eyes of 25 patients with small and mediumsize choroidal
melanoma treated with i-TTT in a retrospective noncomparative interventional
study. Fifteen ml of an aqueous solution containing 75 mg of ICG was administered
as a single IV bolus into a cubital vein, followed by injection of a 10
ml saline flush, 10 minutes before beginning the treatment with the 810
nm IRIS Medical OcuLight® SLx laser. The laser application was initiated
using 60-second exposure duration and 550 mW power over a 3-mm diameter
spot. The power was raised step-wise by 50 to 100 mW steps until the surface
of the tumor developed a grayish color toward the end of the exposure
time. The entire surface of the tumor was treated with confluent 3-mm
diameter sequential applications. A smaller beam diameter was sometimes
used when treating near the macula or the optic disc. Table 1 compares
the tumoral volume before and after the treatment.
Considering the small and medium-size tumors in different analyses, both
groups showed a statistically significant (p <0.05) reduction in tumor
height and volume during the 3 and 6 month follow-up and at the final
visit (length of time, or a range of time). After a mean of 2.4 sessions
of i-TTT (range: 1 to 5) all of the cases, but one, reached the aim of
the therapy, which was to obtain a volume reduction with shrinkage of
the tumoral tissue without clinical evidence of growth during the follow-up.
Fifteen (88%; 15/17) of the small, and four (50%; 4/8) of the medium-size
lesions showed a complete volume involution after treatment and manifested
clinically as flat scar tissue (Figure 1).
Table 1. Results of TTT enhanced with ICG (i-TTT) in choroidal melanomas.

In the rest of the cases, some grade of residual and inactive tumoral
tissue was observed at the final visit. Our experience leads us to believe
that i-TTT may synergistically increase tissue absorption of 810 nm infrared
laser energy and enhance tissue destruction by combined photothermal and
photochemical effects in small and medium-size melanomas as indicated
in experimental and clinical studies. We further believe that i-TTT may
expand the indications for TTT in the treatment of intraocular tumors.
I-TTT IN CHOROIDAL NEOVASCULAR MEMBRANE SECONDARY TO AGE-RELATED
MACULAR DEGENERATION
The additive effect of TTT and ICG could be used with benefit in the management
of choroidal neovascularization (CNV). We herein provide a preliminary
report of our experience in 15 patients with occult CNV using i-TTT as
a reasonable alternative in cases of CNV due to age-related macular degeneration
(AMD) in patients who are not candidates for conventional focal laser
photocoagulation13 or photodynamic therapy (PDT) using Visudyne.14
All the treatments were performed with the IRIS Medical OcuLight SLx laser
delivered to the subretinal neovascular area through the Ocular Mainster
Wide Field contact lens (0.68x image magnification, 1.5x laser beam magnification).
Fifteen ml
of an aqueous solution containing 75 mg of ICG was administered as a single
IV bolus into a cubital vein, followed by injection of a 10 ml saline
flush five minutes before beginning the treatment with the laser. The
laser treatment was performed with a beam diameter of 1.2, 2.0 or 3.0
mm (depending on the diameter of the CNV) for 60 seconds duration at a
power setting ranging from .36 to 1 W (Table 2).
 Table 2. Laser treatment parameters for i-TTT in CNV secondary to
AMD.
Table 2. Laser treatment parameters for i-TTT in CNV secondary to
AMD.
The endpoint was an area of no visible color change to a lightgray appearance.
Care was taken to ensure that the entire lesion border was covered with
the treatment beam. A security area of 500 - 1000 µm from the border of
the lesion was desirable. Immediately after the diode laser application,
ICG angiography was conducted without further ICG administration, and
the sub-visible laser application was confirmed when hypo-fluorescence
of the treated area was observed.
Ten eyes received treatment once and five two times. Occlusion of CNV
and resolution of the exudation were confirmed in 13 of 15 eyes (86.6%)
by the fluorescein angiography (FA) at the final of the follow-up visits
(Figure 2).
In two cases, the CNV occlusion was not completely achieved: The former
remained stable despite that the FA showed a mild escape of dye in late
frames; the latter continued growing after treatment resulting in disciform
scar formation. Because of the presence of scar tissue beneath the fovea,
no additional treatment was recommended. Preoperative visual acuity (VA)
ranged from 6/200 to 20/50 (mean: 20/310). Postoperative VA at the final
follow-up examination ranged from hand motion to 20/30 (mean: 20/336).
VA improved in 4 eyes (26.6%) by more than two lines in the Snellen chart
classification, remained the same in 7 (46.6%), and deteriorated by more
than two lines in 4 (26.6%).
No severe complication occurred during or after the treatment. Nevertheless,
two patients developed a post-treatment choroidal atrophy. Five eyes (33.3%)
required re-treatment for the management of both recurrence (three cases)
and persistence of the CNV (two cases). All the recurrence occurred in
relationship to the border of the membrane. This single-center, retrospective
pilot study examining the use of ICG-enhanced TTT (i-TTT) found potential
short-term VA stabilization in patients with the occult subtype of CNV
in AMD.
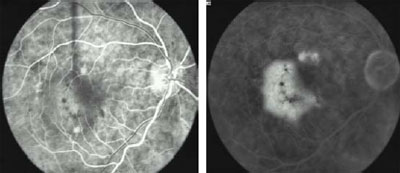 Fig 2a. Pre-treatment. Early FA shows occult, kidney-shaped CNV with
exudative manifestations. VA: 20/50
Fig 2a. Pre-treatment. Early FA shows occult, kidney-shaped CNV with
exudative manifestations. VA: 20/50
Fig 2b. Pre-treatment. Late FA.
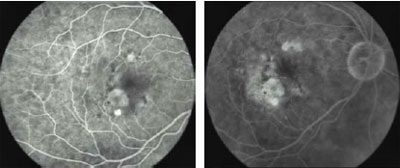 Fig
2c. 3 Months post-treatment. After one session of i-TTT, early FA shows
complete resolution of the exudation. VA: 20/30. Fig
2c. 3 Months post-treatment. After one session of i-TTT, early FA shows
complete resolution of the exudation. VA: 20/30.
Fig 2d. 3 Months post-treatment. Late FA.
REFERENCES
1. Oosterhuris JA, Journee-de Korver HG, Makebeeke-Kemme HM, Bleeker
J. Transpupillary thermotherapy in choroidal melanomas. Arch Ophthalmol
1995;113:315-321.
2. Godfrey DG, Waldron RG, COMT, Capone Jr A. Tranpupillary thermotherapy
for small choroidal melanoma. Am J Ophthalmol 1999;128:88-93.
3. Robertson DM, Buetter H, Bennett SR. Tranpupillary thermotherapy as
primary treatment for small choroidal melanomas. Arch Ophthalmol 1999;117:1512-1519.
4. Shields CL, Shields JA, Perez N, Singh AD, Cater J. Primary transpupillary
thermotherapy for small choroidal melanoma in 256 consecutive cases. Outcomes
and limitations. Ophthalmology 2002;109:225-234.
5. Stoffelns BM. Primary transpupillary thermotherapy (TTT) for malignant
choroidal melanoma. Acta Ophthalmol Scand 2002;80:25-31.
6. Journee-de Korver JG, Verburg-van der Marel EH, Oosterhuris JA, van
Best JA, de Wolff-Rouendaal D. Tumoricidal effect of hyperthermia by near
infrared irradiation on pigmented hamster melanoma. Lasers Light Ophthalmol
1992;4:175-180.
7. Flower RW, Hochheimer BF. Clinical infrared absorption angiography
of the choroid. Am J Ophthalmol 1972;73:458-459.
8. Hope-Ross M, Yannuzzi LA, Gragoudas ES, et al. Adverse reactions due
to indocyanine green. Ophthalmology 1994;101:529-533.
9. Greenwell TJ, Wyman A, Rogers K. Choromophore-enhanced 805 nm laser
therapy for gastrointestinal neoplasia. Eur J Surg Oncol 2001;27: 368-372.
10. Chen WR, Adams RL, Bartels KE, Norquist RE. Chromophore-enhanced in
vivo tumor cell destruction using an 808-nm diode laser. Cancer Lett 1995;94:125-131.
11. Chen WR, Adams RL, Higgins AK, Bartels KE, Norquist RE. Photothermal
effects on murine mammary tumors using indocyanine green and an 808-nm
diode laser: An in vivo efficacy study. Cancer Lett 1996;98: 169-173.
12. Chong LP, Ozler SA, de Queiroz JM Jr, Liggett PE. Indocyanine greenenhanced
diode laser treatment of melanoma in a rabbit model. Retina 1993;13:251-259.
13. Macular Photocoagulation Study Group. Subfoveal neovascular lesions
in age-related macular degeneration. Guidelines for evaluation and treatment
in the macular photocoagulation study. Arch Ophthalmol 1991;109:1242-
1257.
14. Photodynamic therapy of subfoveal choroidal neovascularization in
agerelated macular degeneration with verteporfin: One-year results of
2 randomized clinical trials—TAP report. Treatment of age-related macular
degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol
1999;117:1329-1345.
 La Morita Mission: Eyecare for Those in Need
(Robert N. Johnson, M.D. West Coast Retina, San Francsico, CA) La Morita Mission: Eyecare for Those in Need
(Robert N. Johnson, M.D. West Coast Retina, San Francsico, CA)
Just across the California border in the shadow of San Diego lies Tijuana.
Most people know Tijuana as a kind of cheesy border tourist town. Few
venture to the eastern portions where substantial poverty grows in what
is the fastest growing area of Mexico. Driven by hope of a job, or possibly
crossing the border, Mexicans are migrating to this area in greater numbers.
Approximately 200,000 people live in this region of small towns, or ‘colonias’.
Jobs in local factories pay poorly, and medical care is lacking, or unaffordable.
Houses have dirt floors, no running water, and the roads are unpaved.
These people need help.
In 1997, the La Morita Mission was founded by Fr. Roberto Callahan. Friends
Helping Friends International based in Chula Vista, California, was subsequently
formed to provide assistance in this endeavor. Through dedication, hard
work, and countless hours of volunteer efforts, the San Eugenio Community
Clinic became a reality on August 30, 2003. The Rotary Club in San Diego
has provided a generous grant to equip this medical facility, including
an eye examination room.
My involvement with this clinic has come about through my church’s youth
group. For the last 3 summers, my oldest son has worked at La Morita,
including helping with the construction of the clinic. For me, it has
been particularly rewarding to help with first efforts organizing an eye
care program. The clinic has excellent, but basic equipment. In order
to treat various retinal disorders, access to a laser was essential. I
have used IRIDEX’ IRIS Medical OcuLight GL (532 nm) in the clinic for
a number of years and have experienced its consistent reliability and
ease of use.
The ability to transport this compact laser made it ideal for my work
at La Morita. Air travel with this laser has been quite simple. I have
utilized a small hard-sided photography equipment case made by Pelican.
Using high density foam to cushion the sides, I can transport the laser,
and foot pedal in this smaller case that easily fits in the overhead compartments
on airlines. I have been relieved that carrying this through airport security
has not been a problem, either. I carry the indirect ophthalmoscope attachment
in a separate carry-on with other supplies. I have also utilized on one
of my trips, the OcuLight SL/SLx infrared (810 nm) laser for treatment
of neovascular glaucoma. These seemingly indestructible lasers have been
completely reliable.
My efforts in La Morita are a developing endeavor, but my 5 trips have
affirmed the substantial need. As the prevalence of diabetes is very high,
screening diabetics and treating retinopathy is most common. However,
the pathology is varied, and seeing more advanced disease, such as cases
of vitreous hemorrhage and retinal detachment due to X-linked retinoschisis,
only underscores the need to expand this program further. Currently, I
am unable to perform vitreoretinal surgery, but hope to be able to develop
this aspect of care as well.
 Dr. Johnson at the La Morita Mission, putting the OcuLight GL laser
to use.
Dr. Johnson at the La Morita Mission, putting the OcuLight GL laser
to use.
The personal satisfaction from these efforts has been immense. The dedication
of the sisters and volunteers in the clinic is truly humbling. The patients
are a pleasure to work with and extraordinarily grateful. Although I return
home from these trips tired, I feel that I am the one who has benefitted
from the experience and I look forward to my next trip. Finally, I am
very grateful for the substantial assistance that Eduardo Arias and IRIDEX
has provided to me. The availability of the staff, and assistance with
equipment and advice is truly unique in the industry.
For more information on Friends Helping Friends International, go
to www.fhfinternational.org.
|
 Point-Counterpoint
IV: Surgical Management After Tube-Shunt Failure: Point-Counterpoint
IV: Surgical Management After Tube-Shunt Failure:
What’s the Next Step? Cyclophotocoagulation is an Alternative
(Douglas E. Gaasterland, M.D. University Ophthalmic Consultants of
Washington Chevy Chase, MD)
INTRODUCTION
We treat the intraocular pressure (IOP) in glaucoma with medical and surgical
interventions, with the goal to achieve sufficient reduction in the steady-state
IOP level to inhibit further vision loss.1,2 The IOP reflects the balance
of steadystate aqueous humor inflow and outflow. Steady-state inflow is
relatively IOP independent, equal in most species to about 1% of anterior
chamber volume per minute, in the absence of medications or ciliary surgery.
When relatively high resistance to outflow develops, as in the primary
and secondary open- and closed-angle types of glaucoma, the IOP rises
passively to provide sufficient driving force for outflow, through the
conventional and uveoscleral pathways, to be equal to inflow. Our interventions
for glaucoma enhance outflow (miotics, prostaglandin analogues, various
filtering procedures), reduce inflow (beta blockers, alpha antagonists,
carbonic anhydrase inhibitors, ciliary destructive procedures), or both.
Interventions fail when outflow resistance is so high that IOP rises to
a level threatening the optic nerve and retinal ganglion cells.
Tube-shunt procedures lower IOP by providing an alternative outflow pathway,
reducing outflow resistance. They fail when the resistance remains too
high despite the alternative pathway being present, though there usually
is some outflow. In such a situation, the ophthalmic surgeon has several
options: 1) revise the existing tube-shunt, 2) place another tube-shunt
in a new location, 3) do a standard filtering surgery with adjunctive
antifibrotic in a new location, 4) do a cyclophotocoagulation [or other
procedure to reduce ciliary function], or 5) observe without additional
intervention.
CYCLOPHOTOCOAGULATION—BRIEF HISTORY
There is a nearly 145-year history in ophthalmology of ciliary body surgery
to reduce aqueous inflow (see Bietti3). In 1937, Vogt was first to report
successful penetrating diathermy, a procedure that became widely used
for ciliary ablation.4 This was superseded by cyclocryotherapy in the
1960’s, with the associated success and problems this procedure induces,5
and that was superseded by cyclophotocoagulation for ciliary ablation
after laser-based ophthalmic systems became available in the late 1970’s.
Beckman and co-workers introduced transscleral treatment with continuous-wave
ruby laser in 1972 and with neodymium laser in 1973.6,7 There followed
about 15 years of development of laser systems and procedures for this
purpose with introduction of non-contact and then contact Nd:YAG systems,
and later, smaller, less expensive, portable diode laser-based systems.
With wider availability of diode laser systems, transscleral cyclophotocoagulation
(TSCPC) has become an outpatient, office-based procedure.
CYCLOPHOTOCOAGULATION—DIODE LASER TRANSSCLERAL METHOD
A surgical level of peribulbar, and often retrobulbar, local anesthesia
is required.
Laser energy is delivered from the source by a fiberoptic, preferably
with a spherical tip. The fiber is oriented either perpendicular to the
scleral surface or parallel to the visual axis so that the line of travel
of the light will encounter the ciliary processes inside the eye. These
processes are near the limbus. The spherical tip, which must be optically
clean, indents surface tissues, clearing blood from superficial vessels
in the treatment pathway.
The extent of treatment varies, usually being 3 or 4 quadrants, with about
7 applications per quadrant. Each application is about 4 to 6 J, accomplished
with proper duration of power at 1.25 to 2.5 W. I favor lower power and
longer duration to achieve 5 to 6 J per application. This reduces the
occurrence of audible “pops” during treatment, which are the result of
boiling of water in the target tissue.
INDICATIONS AND RESULTS
There are more than 100 papers in the literature dealing with short to
intermediate-term outcomes after TSCPC for a variety of challenging glaucoma
conditions. The conditions include neovascular glaucoma, recalcitrant
chronic open-angle glaucoma after previous surgery, aphakic glaucoma,
pseudophakic glaucoma, chronic partial or total angle-closure glaucoma,
aniridia, iridocorneal endothelial syndrome, and other lessfrequent problems.
Most of the reports are of case series with a variety of diagnoses, and
some present observations of interesting individual cases. None report
prospective, random assignment comparisons with other methods of management.
In most of these reports, the 1 to 2 year outcome in the series is “successful
control of IOP” in about two-thirds of treated eyes, some of which required
more than one step of ciliary treatment. In the various series of these
eyes with severely threatening glaucoma, there appears to be a 12 - 25%
occurrence of falloff of visual acuity (VA) by two lines or more on the
Snellen chart, with the remainder having VA either unchanged or slightly
improved.
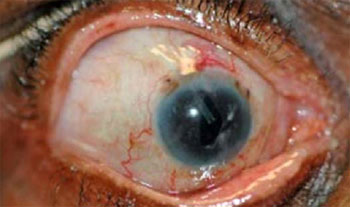 An Illustrative Case - Before: 4 meds, IOP 45. TSCPC performed. This
is 7 months later: 4 meds, IOP 18.
An Illustrative Case - Before: 4 meds, IOP 45. TSCPC performed. This
is 7 months later: 4 meds, IOP 18.
In reviewing many of these papers I found only one study of outcomes of
cyclophotocoagulation after failure of a tube-shunt filtering procedure.
Thus, this article is not aided or hampered by much systematic data. The
one study is by Semchyn, et al., of 21 eyes of 21 patients receiving supplemental
TSCPC after failure of a previous tube-shunt procedure; there was an initial
mean IOP of 35 mm Hg.8 Laser treatment was followed by 7 to 58 months
of follow-up. During this time, 7 eyes needed a second step of laser treatment.
At the end of follow-up, 15 eyes were considered a success, having an
IOP of 21 mm Hg or less, with continued medical treatment. Ten of the
21 eyes had unchanged or improved VA, while the remaining eyes had a falloff
of vision of one or more lines on the Snellen chart.
AN ILLUSTRATIVE CASE
In the past two years of 30 procedures in my office, most for neovascular
glaucoma, I have done only one diode TSCPC treatment for a failed tube
shunt, in a complex situation.
Mr. K was 79 years old when referred for expedited evaluation of an uncomfortable
right eye with IOP in the mid-40’s despite 4 medications. History: Primary
open-angle glaucoma and type II diabetes mellittus x 30 years. Status
post panretinal photocoagulation and scatter photocoagulation for proliferative
diabetic retinopathy and diabetic macular edema. Phacoemulsification with
anterior chamber (AC) intraocular lens (IOL) 4 years ago. Uveitis glaucoma
hyphema (UGH) syndrome. Diode TSCPC. Recurrent UGH. Remove AC IOL. Recurrent
glaucoma. Upper temporal (UT) Ahmed tube-shunt 2 years ago. On exam: VA
count fingers (CF) 1 ft. UT tube. IOP 45. Peripheral anterior synechia
3 quadrants. Foveal scar. I did a repeat diode TSCPC of 25 applications
at 1.25 W and 4.5 seconds to 3 ? quadrants. Mild post-treatment hyphema.
IOP slowly fell to the high teens on 4 medications at 5 months. VA CF
3 ft. Comfortable. At 9 months, IOP was 9 mm Hg and vision unchanged.
CONCLUSION
TSCPC, especially with diode laser systems, due to their more gentle
effects on eye tissue, can reduce inflow sufficiently and effectively,
when combined with continued medical treatment, to bring the aqueous circulation
into balance.
Results from a small number of cases indicate laser TSCPC is an effective
intervention when a tube-shunt procedure fails. For TSCPC to be effective
there is a need for the eye to have some outflow.9 Despite too high an
IOP, eyes with tube-shunt failure probably have some outflow, though insufficient
to balance aqueous humor inflow at a tolerable IOP. TSCPC, especially
with diode laser systems, due to their more gentle effects on eye tissue,
can reduce inflow sufficiently and effectively, when combined with continued
medical treatment, to bring the aqueous circulation into balance.
The American Academy of Ophthalmology panel that reviewed cyclophotocoagulation
in 2001 concluded that the procedure “... is indicated for patients with
refractory glaucoma who have failed trabeculectomy or tube shunt procedures.”10
Data to substantiate the latter are meager.
There is an opportunity and need for a prospective, random assignment
study of management when tube-shunt procedures fail. In at least some
cases, cyclophotocoagulation is helpful. Unfortunately, the magnitude
and duration of improvement and the survival of treated eyes before failure
of cyclophotocoagulation is not yet clear. Thus, there is a paucity of
pilot data to allow formulation of a null hypothesis and calculation of
a sample size for the clinical study.
REFERENCES
1. The AGIS Investigators. The Advanced Glaucoma Intervention Study.
7. The relationship between control of intraocular pressure and visual
field deterioration. Am J Ophthalmol 2000;130:429-40.
2. Heijl A, Leske MC, Bengtsson B, et al. Reduction of intraocular pressure
and glaucoma progression. Results from the Early Manifest Glaucoma Trial.
Arch Ophthalmol 2002;120:1268-79.
3. Bietti G. Surgical interventions on the ciliary body. New trends for
the relief of glaucoma. JAMA 1950;142:889-96.
4. Vogt A. Versuche zur intraokularen Druckherabsetzung mittelst Diathermieschadigung
des Corpus cilare (Zyklodiathermiestichelung). Klin Monatsbl Augenheilkd
1936;97:672-7.
5. Caprioli J, Strang SL, Spaeth GL, Poryzees EH. Cyclocryotherapy in
the treatment of advanced glaucoma. Ophthalmology 1985;92:947-54; with
Discussion by Bellows AR.
6. Beckman H, Kinoshita A, Rota AN, et al. Transscleral ruby laser irradiation
of the ciliary body in the treatment of intractable glaucoma.Trans Am
Acad Ophthalmol Otolaryngol 1972;76:423-35.
7. Beckman H, Sugar HS. Neodymium laser cyclocoagulation. Arch Ophthalmol
1973;90:27-8.
8. Semchyshyn TM, Tsai JC, Joos KM. Supplemental transscleral diode laser
cyclophotocoagulation after aqueous shunt placement in refractory glaucoma.
Ophthalmology 2002;109:1078-1084.
9. Spaeth GA. Discussion of Gaasterland DE and Pollack IP. Initial experience
with a new method of laser transscleral cyclophotocoagulation in severe
glaucoma. Trans Am Ophthalmol Soc 1992;90:225-46.
10. Pastor SA, Singh K, Lee DA, et al. Cyclophotocoagulation. A report
by the American Academy of Ophthalmology. Ophthalmology 2001;108:2130-8.
|
 Did
you Know? Did
you Know?
A NEW CEO ON BOARD On July 5, 2005, IRIDEX’ Board of Directors appointed
Barry Caldwell as its new President and CEO. Ted Boutacoff, who has served
as President and CEO since 1989, has been elected to the position of Chairman
of the Board.
 Barry has more than 30 years of general management, sales, marketing and
corporate business development experience in the ophthalmic industry and
has managed medical device, pharmaceutical and consumer-related products.
He brings to IRIDEX a strong history of execution and an incredible body
of knowledge, relationships, and experience in the ophthalmic industry.
For more information, see the July 5, 2005 press release at www.iridex.com.
Barry has more than 30 years of general management, sales, marketing and
corporate business development experience in the ophthalmic industry and
has managed medical device, pharmaceutical and consumer-related products.
He brings to IRIDEX a strong history of execution and an incredible body
of knowledge, relationships, and experience in the ophthalmic industry.
For more information, see the July 5, 2005 press release at www.iridex.com.
U.S. MARKET
We are pleased to announce the appointment of Robin Drawdy as the Area Sales
Manager for the Southeastern U.S. Robin has extensive experience in ophthalmology
including assignments with Allergan, and most recently, with Laser Diagnostic
Technologies. Robin is based in Atlanta, GA. We are also pleased to welcome
Jeff Zeilinger as the Area Sales Manager for the Mid-Atlantic states. Jeff
joins us after 7 years with Heine USA. Jeff is based in Allentown,PA.
LATIN AMERICA AND CARIBBEAN MARKETS
We are pleased to share the changes that have occurred in our distribution
channels in Latin America. Since attending the Pan American Congress in
Santiago, Chile in March 2005, we have appointed 8 new distributors in Chile,
Brazil, Bolivia, Argentina, Venezuela, Peru, Dominican Republic, and Mexico.
We have also increased our technical service, sales, and market coverage
by the addition of Mr. Gustavo Brescia as Field Service Engineer, International,
who is based in Buenos Aires, Argentina; and Mr. Guillermo Molina as Area
Sales Manager, Latin America, who is based in Miami, FL. These positions
were created to provide direct support to the newly appointed distributors
in Mexico, Central and South America, and the Caribbean. Both gentlemen
have extensive experience in the ophthalmic laser business.
CANADIAN MARKET
We are pleased to announce that Pacific Medical has been appointed IRIDEX’
distributor for the Canadian market. Previously, Pacific Medical was responsible
for IRIS Medical products in Western Canada. Their rapid expansion to cover
Canada opened this opportunity for IRIDEX’ products to be supported by an
excellent organization.
JOINT LICENSING AGREEMENT IRIDEX and Innovatech Surgical
have signed a joint licensing agreement for endophotocoagulation probes.
This agreement gives IRIDEX options for worldwide distribution rights to
Innovatech’s current and future disposable endo ocular probes and Innovatech
will license IRIDEX’ proprietary probe/laser connector. This alliance will
expand IRIDEX’ consumable product line and provide a wider array of laser
probe options to satisfy the demands of vitreoretinal surgeons worldwide.
For more information, see the July 12, 2005 press release at www.iridex.com.
WORLD HEALTH ORGANIZATION In 2004, IRIDEX participated
in the World Health Organization’s (W.H.O.) Elimination of Preventable Child
Blindness Project. This five-year project, started by W.H.O. in 2002, focuses
on training of health personnel for prevention, early detection and treatment;
and establishes child-friendly “Centres for Sight of Children” in 30 countries.
In support of this project, IRIDEX placed multiple OcuLight lasers (810
nm) and laser indirect ophthalmoloscopes in numerous countries worldwide,
including Ethiopia, Lithuania, Romania, Malaysia, Morocco, Philippines,
Indonesia, and Latin America. We are pleased that W.H.O. selected the IRIS
Medical laser systems in support of their efforts to achieve global elimination
of avoidable blindness in children. The OcuLight is well-established worldwide
as the laser of choice for the treatment of retinopathy of prematurity (ROP).
PARENTS’ GUIDE TO ROP IRIDEX partnered with the Association
for Retinopathy of Prematurity and Related Diseases (ROPARD) to published
a parent education brochure on understanding ROP. Tis brochure is available
in English and Spanish and can be ordered directly through ROPARD at www.ropard.org. |
 Список
статей
Список
статей
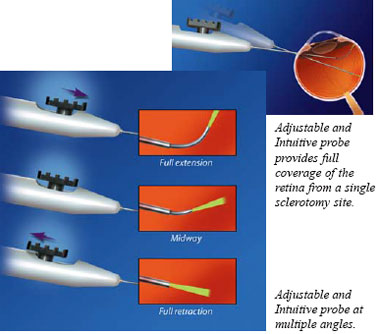 The
fiber optic of the Adjustable and Intuitive probe, can be continuously adjusted
over a wide range of angles (0-90°) for full coverage of the peripheral
retina without removing the probe from the eye. The fiber optic extends
in a logical motion and bends more sharply as the slider is pushed forward,
and offers consistent mode quality and spot size across all angles.
The
fiber optic of the Adjustable and Intuitive probe, can be continuously adjusted
over a wide range of angles (0-90°) for full coverage of the peripheral
retina without removing the probe from the eye. The fiber optic extends
in a logical motion and bends more sharply as the slider is pushed forward,
and offers consistent mode quality and spot size across all angles.  The new Endo Ocular Illuminating probes combine white light illumination
with laser delivery in one convenient handpiece. Its true 20-gauge needle
and bayonet style tip design permits simultaneous wide-field illumination
and precise laser spots. It is compatible with illumination sources from
Alcon, Bausch & Lomb and Synergetics (adapter required).
The new Endo Ocular Illuminating probes combine white light illumination
with laser delivery in one convenient handpiece. Its true 20-gauge needle
and bayonet style tip design permits simultaneous wide-field illumination
and precise laser spots. It is compatible with illumination sources from
Alcon, Bausch & Lomb and Synergetics (adapter required). The 20-24 gauge, 45° angle Stepped EndoProbe permits insertion through
a straight cannula. This offers an alternative to the standard angled probe
for surgeons who prefer to work through a cannula or who don’t use a cannula
but prefer a smaller diameter tip.
The 20-24 gauge, 45° angle Stepped EndoProbe permits insertion through
a straight cannula. This offers an alternative to the standard angled probe
for surgeons who prefer to work through a cannula or who don’t use a cannula
but prefer a smaller diameter tip. The existing family of IRIS Medical probes includes 20 gauge: straight,
passive aspirating, active aspirating, angled, and illuminating models;
and 25 gauge straight.
The existing family of IRIS Medical probes includes 20 gauge: straight,
passive aspirating, active aspirating, angled, and illuminating models;
and 25 gauge straight. Barry has more than 30 years of general management, sales, marketing and
corporate business development experience in the ophthalmic industry and
has managed medical device, pharmaceutical and consumer-related products.
He brings to IRIDEX a strong history of execution and an incredible body
of knowledge, relationships, and experience in the ophthalmic industry.
For more information, see the July 5, 2005 press release at www.iridex.com.
Barry has more than 30 years of general management, sales, marketing and
corporate business development experience in the ophthalmic industry and
has managed medical device, pharmaceutical and consumer-related products.
He brings to IRIDEX a strong history of execution and an incredible body
of knowledge, relationships, and experience in the ophthalmic industry.
For more information, see the July 5, 2005 press release at www.iridex.com.



 Fig 1c. Red free fundus
Fig 1c. Red free fundus Fig 1d. Middle FA frame 3 months after the second session disclosed
residual tissue without evidence of activity. BCVA: 20/60.
Fig 1d. Middle FA frame 3 months after the second session disclosed
residual tissue without evidence of activity. BCVA: 20/60.

 Table 2. Laser treatment parameters for i-TTT in CNV secondary to
AMD.
Table 2. Laser treatment parameters for i-TTT in CNV secondary to
AMD. Fig 2a. Pre-treatment. Early FA shows occult, kidney-shaped CNV with
exudative manifestations. VA: 20/50
Fig 2a. Pre-treatment. Early FA shows occult, kidney-shaped CNV with
exudative manifestations. VA: 20/50 Fig
2c. 3 Months post-treatment. After one session of i-TTT, early FA shows
complete resolution of the exudation. VA: 20/30.
Fig
2c. 3 Months post-treatment. After one session of i-TTT, early FA shows
complete resolution of the exudation. VA: 20/30. Dr. Johnson at the La Morita Mission, putting the OcuLight GL laser
to use.
Dr. Johnson at the La Morita Mission, putting the OcuLight GL laser
to use. An Illustrative Case - Before: 4 meds, IOP 45. TSCPC performed. This
is 7 months later: 4 meds, IOP 18.
An Illustrative Case - Before: 4 meds, IOP 45. TSCPC performed. This
is 7 months later: 4 meds, IOP 18.